Demystifying the “Red” in Red Velvet Cake
Three sentences NEVER came out of my mouth:
“I wish this cake tasted less chocolaty.”
“You know what chocolate cake needs… cream cheese!”
“Red food coloring makes everything better.”
In short, I don’t like Red Velvet Cake. It tastes like it’s trying to be something other than a chocolate cake, but can’t decide what. That said, it offers a fascinating demonstration of chemistry, and an insight into why certain ingredients frequently appear together.
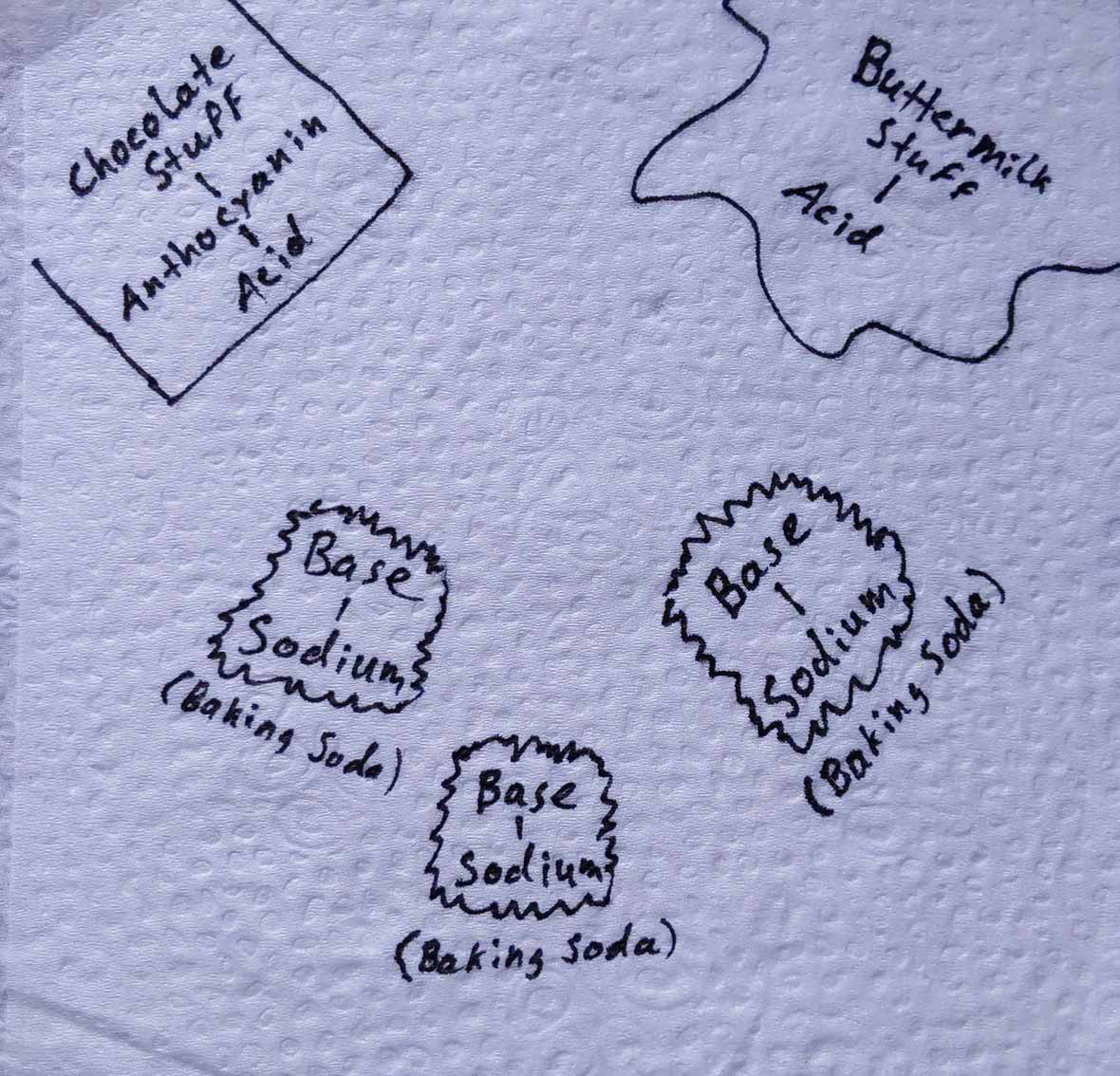
In the early days, Red Velvet Cake enjoyed a subtle red color not caused by food coloring. The color resulted from a chemical reaction between buttermilk, baking soda, and an unprocessed cocoa powder containing anthocyanins.
Anthocyanins represent a group of powerful antioxidants found in many red or purple foods such as red cabbage, pomegranates, blueberries, raspberries, and dark chocolate. It turns out they’re also pH indicators. In other words, they change color depending on the acidity of the environment. In acids they become red, and in bases they become greenish.
The acidic cocoa and buttermilk in Red Velvet batter react with the baking soda which is a base (figure 1).
The acid-base reaction forcibly pulls apart and redistributes the molecular groups in the batter (figure 2).

Among the byproducts of the reaction are water, carbon dioxide and anthocyanin, which all now freely move about in the batter (figure 3).
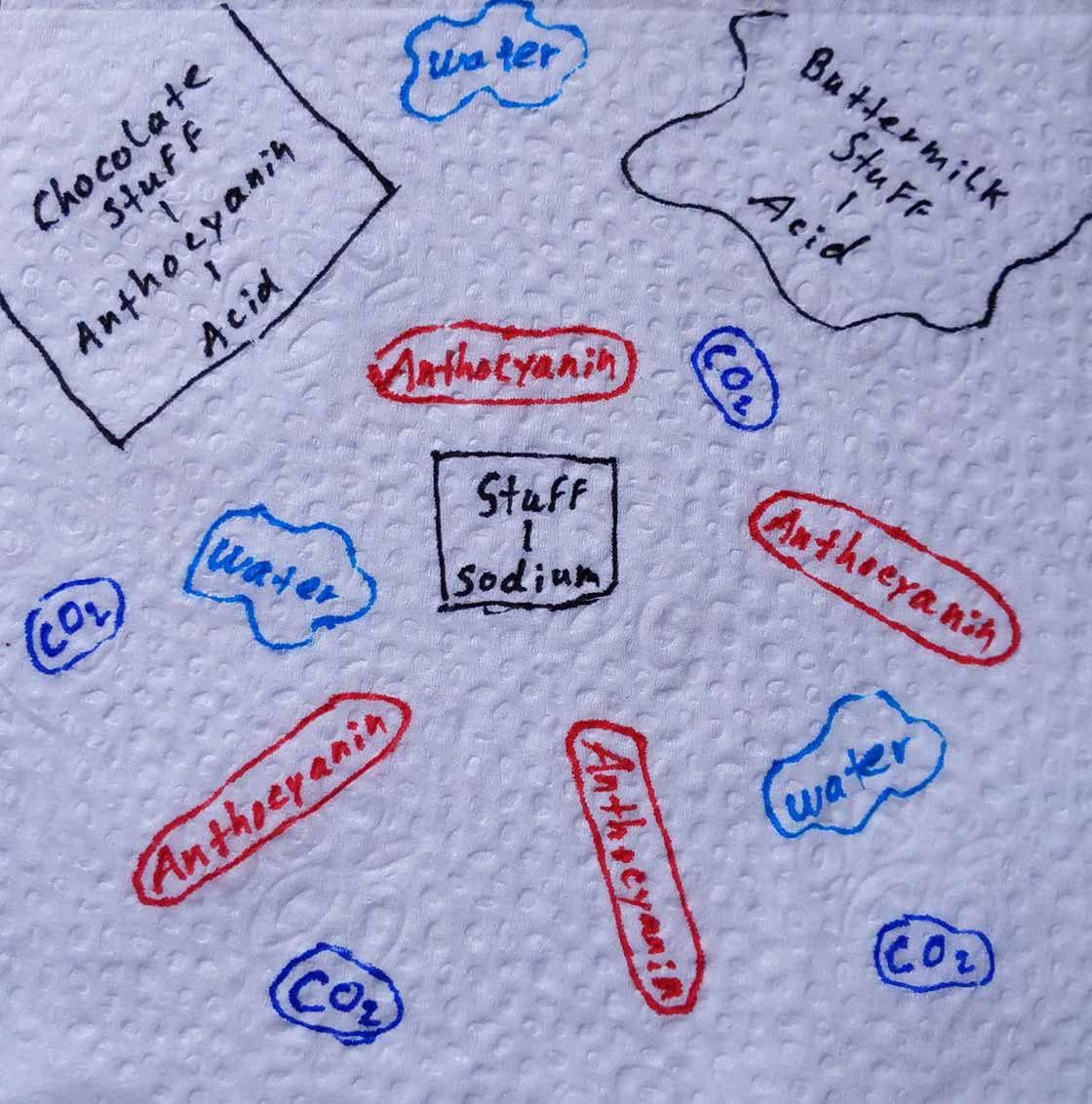
Since the batter is on the acidic side, the anthocyanin turns red and colors the cake as it cooks. These cakes weren’t as vibrantly red as the ones we see now, but still managed an elegant maroon hue.
Next time you decide to make a Red Velvet Cake, set aside the food coloring and give it a chance to make the statement, “Chemistry is awesome, even if this cake isn’t!”
Science TidbitAcid-base reactions do much more than release pH indicators into food, they primarily support the creation of the gas needed for cakes and cookies to rise. Carbon Dioxide is usually the goal when recipes involve Baking Soda or Baking Powder. That CO2 expands in the oven causing baked goods to rise, contributing to the formation of a good ‘crumb’.


 Next Post
Next Post